Improving the stability and inertness of Cu(ii) and Cu(i) complexes with methylthiazolyl ligands by tuning the macrocyclic structure†
Abstract
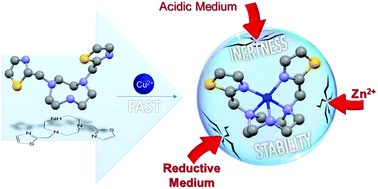
A tacn based ligand bearing two methylthiazolyl arms (no2th) was synthesized with the aim to find ligands forming very stable and inert complexes with Cu(II) and Cu(I) in aqueous medium for radiopharmaceutical applications. The no2th ligand was efficiently prepared following the orthoamide intermediate synthesis. The complexes with Cu2+ and Zn2+ were obtained and analyzed by X-ray diffraction. The [Cu(no2th)]2+ complex presents a pentacoordinated distorted square pyramidal coordination geometry, while the metal ion in [Zn(no2th)]2+ adopts a hexacoordinated distorted trigonal prismatic geometry involving the coordination of a perchlorate counter ion. The acid–base properties of no2th have been studied using potentiometric titrations, and the stability constants of Cu2+ and Zn2+ complexes were determined by potentiometric and UV-vis titrations using H4edta as a competitor ligand. The stability constant determined for the Cu2+ complex is rather high (log KCuL = 20.77 and pCu = 17.15), and moreover no2th exhibits a high selectivity for copper(II) in relation to zinc(II). The kinetics of the copper(II) complexation process is very fast even in acidic medium. In addition, the [Cu(no2th)]2+ complex was found to be inert under rather harsh conditions (up to 2 M HCl and 60 °C), displaying a very high half-life time of about 15 days in 2 M HCl at 90 °C. The electrochemical reduction of the copper(II) complex in water leads to the reversible formation of a stable copper(I) species. Spectroscopic studies performed by NMR, UV-vis and EPR, assisted by theoretical calculations, show that the [Cu(no2th)]2+ complex presents a structure in solution similar to that observed in the solid state. When compared to its cyclam di-N-methylthiazolyl counterpart, the results reported in this paper unambiguously show that replacing the cyclam unit by a tacn moiety improves the stability and inertness of its Cu(II) and Cu(I) complexes.

 Please wait while we load your content...
Please wait while we load your content...