Electrochemistry and catalytic properties of amphiphilic vitamin B12 derivatives in nonaqueous media†
Abstract
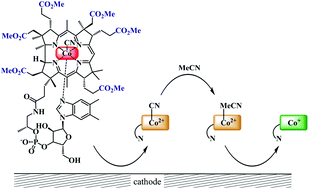
The reduction pathway of cobalester (CN)Cble, an amphiphilic vitamin B12 derivative, was investigated in organic solvents under electrochemical conditions and compared with mono- and dicyanocobyrinates. The redox characteristics were determined using cyclic voltammetry and spectroelectrochemical methods. The presence of a nucleotide moiety in B12-derivative impedes the in situ formation of dicyano-species thus facilitating the (CN)Co(III) to Co(I) reduction. The (CN)Cble shows stepwise reduction to Co(I) via (CN)Co(II). The reduction of (CN)Co(II)/Co(I) was found to depend on cyanide-solvent exchange equilibrium with weakly coordinating solvents and bulky peripheral chains promoting intact (CN)Co(II) species existence. The studied complexes were also utilized as catalysts in bulk electrolysis of benzyl bromide affording bibenzyl in very good yield.

 Please wait while we load your content...
Please wait while we load your content...