Cyclopentadienyl nickel(ii) N,C-chelating benzothiazolyl NHC complexes: synthesis, characterization and application in catalytic C–C bond formation reactions†
Abstract
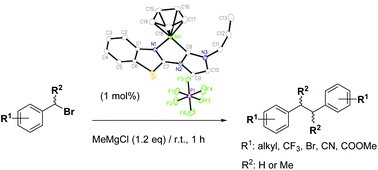
Cyclopentadienyl (Cp) Ni(II) complexes [CpNiL][PF6] containing hybrid N,C chelating benzothiazolyl NHC ligands (L1 = 1-(2-benzothiazolyl)-3-methylimidazol-2-ylidene, 3a; L2 = 1-(2-benzothiazolyl)-3-allylimidazol-2-ylidene, 3b; L3 = 1-(2-benzothiazolyl)-3-benzylimidazol-2-ylidene, 3c) have been synthesized and fully characterized. The catalytic activity of 3a–3c in some C–C bond formation reactions has been examined. They are efficient catalysts for the homo-coupling of benzyl bromide in the presence of MeMgCl at r.t. with good functional group tolerance. Complex 3a is active in the catalytic oxidative homo-coupling of Grignard reagents with 1,2-dichloroethane as an oxidant at r.t.

 Please wait while we load your content...
Please wait while we load your content...