Probing the reactivity of pentaphenylborole with N–H, O–H, P–H, and S–H bonds†
Abstract
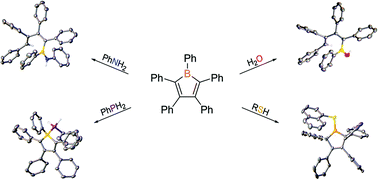
The reactions of molecules containing E–H functionalities (E = Group 15 or 16 element) and pentaphenylborole were investigated revealing diverse outcomes. For aniline and water, protodeborylation ring opening reactions occurred via the N–H or O–H bonds. Pentaphenylborole reacted with water in a 1 : 1 or 2 : 1 ratio to yield the corresponding boroxane and diboroxane, respectively, whereas aniline reacted strictly in a 1 : 1 ratio. Interestingly, 1-naphthalenethiol reacted to produce a 1-bora-cyclopent-3-ene heterocycle. The reaction with a primary phosphine generated an adduct which was resilient, even at elevated temperatures. DFT calculations provide support for the observed reaction products, and identify the initial adduct as a key intermediate in determining the final product. In particular, ring opening may be linked to the lability of the hydrogen in the initial adduct. Collectively, these reactions provide insight into new reaction pathways, the stability of boroles, as well as mechanistic insight into previously reported transformations.
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...