Transformation of zinc hydroxide chloride monohydrate to crystalline zinc oxide†
Abstract
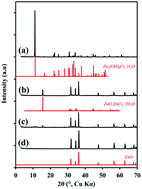
Thermal decomposition of layered zinc hydroxide double salts provides an interesting alternative synthesis for particles of zinc oxide. Here, we examine the sequence of changes occurring as zinc hydroxide chloride monohydrate (Zn5(OH)8Cl2·H2O) is converted to crystalline ZnO by thermal decomposition. The specific surface area of the resultant ZnO measured by BET was 1.3 m2 g−1. A complicating and important factor in this process is that the thermal decomposition of zinc hydroxide chloride is also accompanied by the formation of volatile zinc-containing species under certain conditions. We show that this volatile compound is anhydrous ZnCl2 and its formation is moisture dependent. Therefore, control of atmospheric moisture is an important consideration that affects the overall efficiency of ZnO production by this process.

 Please wait while we load your content...
Please wait while we load your content...