Aminophobanes: hydrolytic stability, tautomerism and application in Cr-catalysed ethene oligomerisation†
Abstract
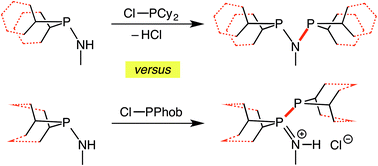
9-Amino-9-phosphabicyclo[3.3.1]nonanes, (PhobPNHR′; R = Me or iPr) are readily prepared by aminolysis of PhobPCl and are significantly less susceptible to hydrolysis than the acyclic analogues Cy2PNHR′. Treatment of Cy2PNHMe with Cy2PCl readily gave Cy2PNMePCy2. By contrast, treatment of PhobPCl with PhobPNHMe in the presence of Et3N does not afford PhobPNMePPhob but instead the salt [PhobP(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMeH)PPhob]Cl is formed which, upon addition of [PtCl2(NCtBu)2] gives the zwitterionic complex [PtCl3(PhobP(
NMeH)PPhob]Cl is formed which, upon addition of [PtCl2(NCtBu)2] gives the zwitterionic complex [PtCl3(PhobP(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMeH)PPhob)]. The neutral PhobP(
NMeH)PPhob)]. The neutral PhobP(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMe)PPhob is accessible from PhobNMeLi and is converted to the chelate [PdCl2(PhobPNMePPhob)] by addition of [PdCl2(cod)]. The anomalous preference of the PhobP group for the formation of PPN products is discussed. The unsymmetrical diphos ligands PhobPNMePAr2 (Ar = Ph, o-Tol) are prepared, converted to [Cr(CO)4(PhobPNMePAr2)] and shown to form Cr-catalysts for ethene oligomerisation, producing a pattern of higher alkenes that corresponds to a Schulz-Flory distribution overlaid on selective tri/tetramerisation.
NMe)PPhob is accessible from PhobNMeLi and is converted to the chelate [PdCl2(PhobPNMePPhob)] by addition of [PdCl2(cod)]. The anomalous preference of the PhobP group for the formation of PPN products is discussed. The unsymmetrical diphos ligands PhobPNMePAr2 (Ar = Ph, o-Tol) are prepared, converted to [Cr(CO)4(PhobPNMePAr2)] and shown to form Cr-catalysts for ethene oligomerisation, producing a pattern of higher alkenes that corresponds to a Schulz-Flory distribution overlaid on selective tri/tetramerisation.
- This article is part of the themed collection: Phosphorus Chemistry: Discoveries and Advances

 Please wait while we load your content...
Please wait while we load your content...