Tris(pyrazolyl)phosphines with copper(i): from monomers to polymers†
Abstract
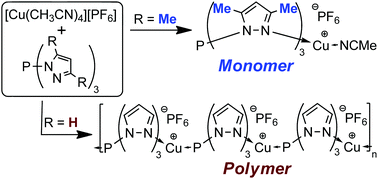
The parent tris(pyrazolyl)phosphine and its 3,5-Me2, 3-Ph, and 3-t-Bu derivatives have been prepared by a simple procedure and show modest Lewis basicity of the phosphorus apex as was established by the magnitude of the 1JP,Se coupling constant of the phosphine selenides. Because of the chelating properties of both the N- and P-sites, neutral phosphorus-centered scorpion ligands allow coordination modes that are unavailable to the abundantly used anionic tris(pyrazolyl)borate scorpionates as we established for Cu(I)-complexation. The substituted P-scorpion ligands only allow for N-coordination, as the P-apex is presumably less accessible. Two X-ray crystal structures were obtained for the Cu-complex of tris(3,5-dimethylpyrazolyl)-phosphine with acetonitrile and triphenylphosphine in the fourth coordination site. The parent P-scorpion ligand can chelate with both its pyrazolyl groups and its P-apex with the product depending on the ratio in which it is mixed with the Cu(I) complex. Reacting two equivalents of the ligand with [Cu(MeCN)4][PF6] resulted in a complex in which Cu is coordinated to the three pyrazolyl groups of one ligand and to the P-apex of the other ligand as confirmed by an X-ray crystal structure determination and a DFT computational analysis. Reacting the ligand and the Cu(I) complex in an equimolar ratio resulted in a remarkable one-dimensional P-scorpion coordination polymer for which a single crystal X-ray structure could be determined. A detailed analysis of the structural features is presented.
- This article is part of the themed collection: Phosphorus Chemistry: Discoveries and Advances


 Please wait while we load your content...
Please wait while we load your content...