First structural characterization of Pa(iv) in aqueous solution and quantum chemical investigations of the tetravalent actinides up to Bk(iv): the evidence of a curium break†
Abstract
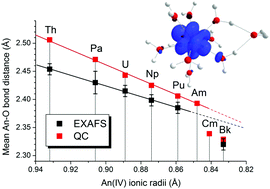
More than a century after its discovery the structure of the Pa4+ ion in acidic aqueous solution has been investigated for the first time experimentally and by quantum chemistry. The combined results of EXAFS data and quantum chemically optimized structures suggest that the Pa4+ aqua ion has an average of nine water molecules in its first hydration sphere at a mean Pa–O distance of 2.43 Å. The data available for the early tetravalent actinide (An) elements from Th4+ to Bk4+ show that the An–O bonds have a pronounced electrostatic character, with bond distances following the same monotonic decreasing trend as the An4+ ionic radii, with a decrease of the hydration number from nine to eight for the heaviest ions Cm4+ and Bk4+. Being the first open-shell tetravalent actinide, Pa4+ features a coordination chemistry very similar to its successors. The electronic configuration of all open-shell systems corresponds to occupation of the valence 5f orbitals, without contribution from the 6d orbitals. Our results thus demonstrate that Pa(IV) resembles its early actinide neighbors.

 Please wait while we load your content...
Please wait while we load your content...