A discrete {Co4(μ3-OH)4}4+ cluster with an oxygen-rich coordination environment as a catalyst for the epoxidation of various olefins†
Abstract
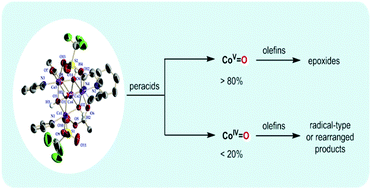
Using the sterically hindered terphenyl-based carboxylate, the tetrameric Co(II) complex [Co4(μ3-OH)4(μ-O2CAr4F-Ph)2(μ-OTf)2(Py)4] (1) with an asymmetric cubane-type core has been synthesized and fully characterized by X-ray diffraction, UV-vis spectroscopy, and electron paramagnetic resonance spectroscopy. Interestingly, the cubane-type cobalt cluster 1 with 3-chloroperoxybenzoic acid as the oxidant was found to be very effective in the epoxidation of a variety of olefins, including terminal olefins which are more challenging targeting substrates. Moreover, this catalytic system showed a fast reaction rate and high epoxide yields under mild conditions. Based on product analysis and Hammett studies, the use of peroxyphenylacetic acid as a mechanistic probe, H218O-exchange experiments, and EPR studies, it has been proposed that multiple reactive cobalt-oxo species CoV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and CoIV
O and CoIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O were involved in the olefin epoxidation.
O were involved in the olefin epoxidation.

 Please wait while we load your content...
Please wait while we load your content...