Benzoindolium–triarylborane conjugates: a ratiometric fluorescent chemodosimeter for the detection of cyanide ions in aqueous medium†
Abstract
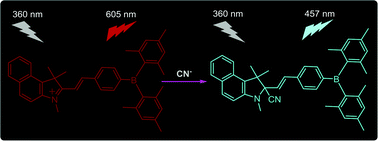
Based on benzo[e]indolium and dimesitylborylbenzaldehyde a new ratiometric fluorescent chemodosimeter, C41H43BIN (3) has been synthesized and characterized by 1H, 13C NMR spectroscopy, mass spectrometry and single crystal X-ray crystallography. Probe 3 was found to be highly selective and sensitive toward cyanide (CN−) ions in aqueous medium even in the presence of other competing anions like F−, Cl−, Br−, I−, H2PO4−, HCO3− and AcO−. The detection limit was calculated to be 7.1 × 10−9 M, which is much lower than the maximum permissable concentration in drinking water (1.9 μM) set by the World Health Organization (WHO). In addition, the response time of the probe for CN− is less than 5 seconds. The mechanism is based on a nucleophilic addition reaction of cyanide ions at the polarized [>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N<]+ bond of the benzoindolium group thereby blocking the pi-conjugation between the benzoindolium and triarylborane moiety. This was further confirmed by 1H NMR titration, ESI-MS studies and DFT calculations.
N<]+ bond of the benzoindolium group thereby blocking the pi-conjugation between the benzoindolium and triarylborane moiety. This was further confirmed by 1H NMR titration, ESI-MS studies and DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...