Abstract
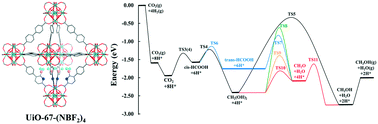
Capture and conversion of CO2 to methanol using a renewable source of H2 is a promising way to reduce net CO2 emissions while producing valuable fuels. Design of an efficient heterogeneous catalyst for CO2 hydrogenation is critical for the large-scale implementation of the CO2 to methanol process. Combining both capture and conversion of CO2 in a single material has the potential to reduce the overall cost through process intensification. We have used density functional theory to computationally design a catalyst capable of producing methanol from CO2 and H2, including calculating the reaction pathways and barriers of each step. The catalyst consists of a microporous metal organic framework (UiO-67) functionalized with catalytically active Lewis pair functional groups. Our calculations indicate that this novel catalyst facilitates the heterolytic dissociation of H2 to generate hydridic and protic H atoms bound to Lewis acid and base sites, respectively, which facilitates a series of simultaneous transfer of two hydrogens to produce methanol: 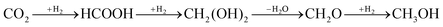 . Importantly, our catalyst binds H2 more strongly than CO2, which prevents CO2 from poisoning the Lewis acid and base sites.
. Importantly, our catalyst binds H2 more strongly than CO2, which prevents CO2 from poisoning the Lewis acid and base sites.


 Please wait while we load your content...
Please wait while we load your content...