Phenol hydrodeoxygenation: effect of support and Re promoter on the reactivity of Co catalysts†
Abstract
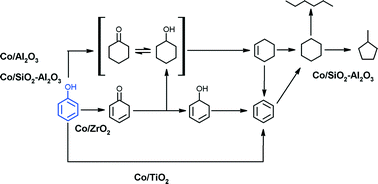
In this work, the reactivity of supported Co catalysts as a function of the oxide support (alumina, silica-alumina, zirconia and titania) and Re promoter for the hydrodeoxygenation of phenol at 300 °C and 3 MPa H2 using a batch autoclave reactor was investigated. The catalyst properties have been obtained from N2 physisorption, X-ray Diffraction (XRD), Atomic Absorption Spectroscopy (AAS), Temperature-Programmed Reduction (TPR), Temperature-Programmed Desorption of ammonia (NH3-TPD), H2 chemisorption and X-ray Photoelectron spectroscopy (XPS). Characterization results revealed the presence of Co particles existing as Co metal and CoO whose surface properties were controlled by the surface behavior of the oxide supports. It was found that phenol conversion proceeded via three major pathways which were dictated by the support. The Co/Al2O3 and Co/SiO2–Al2O3 catalysts preferred the well-documented sequential ring hydrogenation–dehydration–hydrogenation (or dehydrogenation) route while phenol tautomerization, followed by hydrogenation and dehydration was observed in the case of the Co/ZrO2 catalyst. The catalytic activity was governed by a combination of metal sites and acid properties of metal–support interface and decreased in the order: Co/Al2O3 > Co/SiO2–Al2O3 > Co/ZrO2 > Co/TiO2. The markedly low activity of the Co/TiO2 catalyst was attributed to the presence of a thin TiOx layer which partially covered the active metal sites due to the strong metal–support interaction effect. The low activity, however, is offset by this catalyst's ability to catalyze the efficient production of benzene directly from phenol. The addition of Re to the supported Co catalysts had a beneficial effect on the activity, attributed to improved reducibility and the presence of additional hydrogenation sites. The strongest effect of Re addition on the activity and selectivity was observed for the TiO2-supported catalyst. The results further highlight the importance of the choice of support in HDO reactions.

 Please wait while we load your content...
Please wait while we load your content...