Depolymerization of 1,4-polybutadiene by metathesis: high yield of large macrocyclic oligo(butadiene)s by ligand selectivity control†
Abstract
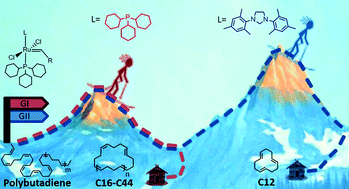
Herein, we demonstrate a practical high yield preparation of large macrocyclic oligo(butadiene)s, preferably the C16 to C44 fraction, from commercial 1,4-polybutadiene by exploring intramolecular backbiting using a series of commercially available Ru catalysts. Product contamination with linear fragments is restricted by using high molecular weight 1,4-polybutadiene with a low content of 1,2-constructs (vinyl groups). The distribution of the cyclic compounds is largely dependent on the nature of the ligand structure of the Ru catalyst. Kinetic inspection of the reaction reveals a two-step mechanism involving (i) backbiting of the linear polymer with initial formation of large macrocycles followed by (ii) tandem ring-opening ring-closing metathesis predominantly leading to thermodynamically favorable t,t,t-cyclododecatriene (CDT). In particular, second-generation Ru catalysts with N-heterocyclic carbene (NHC) ligands favor undesired CDT formation. First-generation catalysts, presumably due to their high barriers for formation of the intermediate metallacyclobutane, selectively form the C16 to C44 macrocyclic oligo(butadiene) fraction. For example, reaction of (HMW, 98% cis)-polybutadiene with a first-generation Ru catalyst almost yields 90% C16–C44 cyclic oligo(butadiene)s.

 Please wait while we load your content...
Please wait while we load your content...