Bisphenol A degradation by a new acidic nano zero-valent iron diatomite composite
Abstract
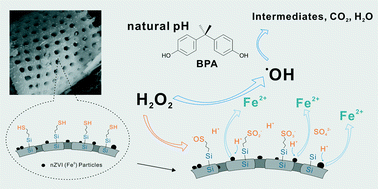
A new nano zero-valent iron (nZVI) material that generates acidic conditions in situ was prepared by grafting an acid precursor onto diatomite. This new material was used in the evaluation of Bisphenol A (BPA) degradation by a Fenton-like process. The prepared material (M-nZVI-Da) exhibited a high removal efficiency (100%) of BPA under natural pH conditions of the solution (pH ∼5.75). The degradation of BPA using this new catalyst material follows a reaction pathway modelled on Fermi's equation with predictable kinetic outcomes for increased temperature of the reaction. Experiments demonstrate that the optimum starting concentration ratio of the H2O2/sample is 200 mM g−1 for efficient catalyst use. This study shows that nZVI materials with acid precursors are efficient catalysts for removal of BPA in solution.


 Please wait while we load your content...
Please wait while we load your content...