QM/MM study of the mechanism of reduction of 3-hydroxy-3-methylglutaryl coenzyme A catalyzed by human HMG-CoA reductase†
Abstract
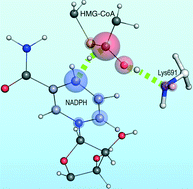
Detailing with atomistic resolution the reaction mechanism of human HMG-CoA reductase (HMG-CoA-R) might provide valuable insights for the development of new cholesterol-lowering drugs. In the pursuit of that goal we developed three molecular models of human HMG-CoA-R with different active site protonation states and employed molecular dynamics (MD) and quantum mechanics/molecular mechanics (QM/MM) calculations to detail the first reduction step, the rate-limiting step, of HMG-CoA-R. Our results predict an active site with a neutral glutamate (Glu559) as the most catalytically competent structure. The favored reaction pathway suggests the formation of a mevaldyl-CoA intermediate protonated by a conserved active site lysine (Lys691), corroborating previous site-directed mutagenesis studies. The conserved active site glutamate and aspartate residues (Glu559 and Asp767), along with the ribose moiety of NADPH, form a hydrogen bond network crucial to the increase of the stabilizing effect of Lys691 over the transition state.

 Please wait while we load your content...
Please wait while we load your content...