The interaction of reactants, intermediates and products with Cu ions in Cu-SSZ-13 NH3 SCR catalysts: an energetic and ab initio X-ray absorption modeling study†
Abstract
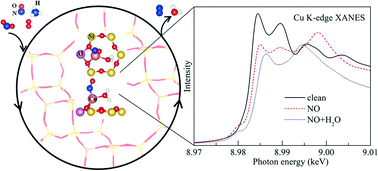
In this contribution, the most likely positions for Cu in Cu-SSZ-13 with a single charge compensating Al atom (ZCu) with a Si : Al ratio of 11 : 1 were investigated, including the effect of the adsorption of reactants, intermediates, and products that one would find in an NH3 SCR reaction by using first-principles calculations based on density functional theory. The 6-membered ring (6MR) site is the most energetically favorable, while the 8-membered ring (8MR) sites are less favorable with energy differences of about 0.5 eV with respect to the 6MR site for plain ZCu. Upon molecular adsorption, the energy differences between Cu in the 8MR and 6MR sites decrease and, in some cases, almost disappear. For the complex scenarios of NO or CO adsorption, the co-adsorption of 2 NO or 2 CO molecules, as well as NO or CO with OH and H2O, weakens the interaction between adsorbates and Cu. The X-ray absorption near edge structure (XANES) of Cu in Cu-SSZ-13 under different conditions was also modeled from first principles. A small peak feature around 8979.5 eV was found in the K-edge XANES of Cu for a clean ZCu conformation with Cu in the 8MR site, while this feature is absent when Cu is in the 6MR site. We correlate this result for this case as well as for other representative configurations by analyzing the corresponding PDOS of the excited state of Cu while taking into account the core-hole effect. Molecular adsorption onto Cu in the 6MR or 8MR site results in a Cu K-edge XANES that is independent of its location. When NO (or CO, N2) is adsorbed onto Cu in ZCu, a small peak feature in the K-edge XANES appears at 8980 eV, which is induced by the splitting of the Cu 4p state. An analysis of the XANES in the presence of two co-adsorbed species on an isolated Cu ion shows that the K-edge position of Cu has the following order (from low to high energy): clean < M < M + H2O < 2M < M + OH (M denotes NO or CO). As a result, we conclude that (1) XANES can readily distinguish between adsorbates on ZCu due to their different oxidizing capacities, but (2) XANES cannot be used to distinguish between the Cu location and the oxidation state in the presence of adsorbates, except in the presence of H2O and NH3 where such distinctions can be made.


 Please wait while we load your content...
Please wait while we load your content...