Gas-phase cascade upgrading of furfural to 2-methylfuran using methanol as a H-transfer reactant and MgO based catalysts†
Abstract
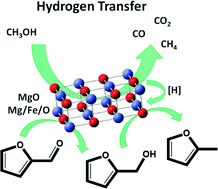
The hydrogenation of biomass-derived molecules is a key reaction in upgrading these compounds into chemicals and fuels. The use of catalytic transfer hydrogenation, employing alcohols as hydrogen sources, offers an alternative approach to this process, avoiding the use of H2 under high pressure and precious metal catalysts. In this work, the gas-phase conversion of biomass-derived furfural into furfuryl alcohol and 2-methylfuran was studied, using methanol as the H-transfer agent and MgO-based catalysts. Pure MgO was shown to reduce furfural into its corresponding unsaturated alcohol at low reaction temperatures (lower than 350 °C), thus allowing selective H-transfer from methanol to the substrate. 2-Methylfuran formation, associated with the partial rearrangement of furan rings to cyclopentanones, was observed at high temperatures. Conversely, the distribution of compounds obtained with Mg/Fe/O was significantly different, with 2-methylfuran formation prevailing when the reaction was carried out between 300 and 400 °C. In this temperature range, upon tuning the reaction conditions, a very high yield of 2-methylfuran was produced, thus indicating that the mixed oxide allows efficient sequential transfer hydrogenation/hydrogenolysis reactions. These results highlight the potential application of the H-transfer reaction over MgO-based catalysts as an efficient process for the selective de-oxygenation of biomass-derived molecules.

 Please wait while we load your content...
Please wait while we load your content...