Reversible cyclometalation at RhI as a motif for metal–ligand bifunctional bond activation and base-free formic acid dehydrogenation†
Abstract
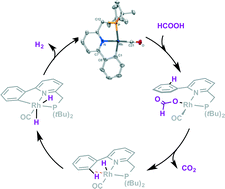
Reversible cyclometalation is demonstrated as a strategy for the activation of small protic molecules, with a proof-of-principle catalytic application in the dehydrogenation of formic acid in the absence of an exogenous base. The well-defined RhI complex Rh(CO)(L) 1, bearing the reactive cyclometalated PN(C) ligand L (LH = PNCH = 2-di(tert-butylphosphinomethyl)-6-phenylpyridine), undergoes protonolysis of the Rh–CPh bond with weak protic reagents, such as thiols and trifluoromethanesulfonamide. This system also displays bifunctional metal–ligand protonolysis reactivity with formic acid and subsequent decarboxylation of the formate complex. Density functional theory (DFT) calculations show that H2 evolution from putative Rh(CO)(H)(LH) complex A is very facile, proposedly encompassing formal C–H oxidative addition at Rh to give Cvia agostic intermediate B and subsequent reductive elimination of H2. Complex 1 is a catalytically competent species for base-free formic acid dehydrogenation, with the intermediacy of formate complex 4. DFT calculations reveal accessible barriers for involvement of a flanking phenyl group for both initial activation of formic acid and release of H2, supporting a cooperative pathway. Reversible C–H activation is thus a viable mechanism for metal–ligand bifunctional catalysis.
- This article is part of the themed collection: 2016 most accessed Catalysis Science and Technology articles

 Please wait while we load your content...
Please wait while we load your content...