Hydrogenation of CO2 to formic acid with iridiumIII(bisMETAMORPhos)(hydride): the role of a dormant fac-IrIII(trihydride) and an active trans-IrIII(dihydride) species†
Abstract
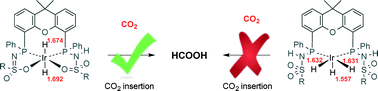
An IrIII-monohydride species bearing a chemoresponsive ligand is active in catalytic CO2 hydrogenation to formic acid with DBU as the exogenous base. Spectroscopic and computational data reveal a trans-IrIII-dihydride as the essential catalytic intermediate and an IrIII(H)3 species as the dormant off-cycle product. This insight will aid future design of improved CO2 reduction catalysts.
- This article is part of the themed collection: 2016 most accessed Catalysis Science and Technology articles

 Please wait while we load your content...
Please wait while we load your content...