Cyclopentadienyl-based Mg complexes in the intramolecular hydroamination of aminoalkenes: mechanistic evidence for cationic versus neutral magnesium derivatives†
Abstract
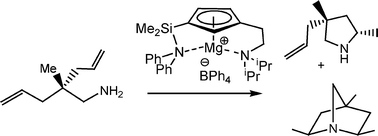
Neutral and cationic magnesium complexes stabilized by coordination of a cyclopentadienyl ligand with two different neutral hemilabile donor groups [Mg{η5-C5H3-1,3-(CH2CH2NiPr2)(SiMe2NPh2)}X(thf)] (X = Bz (2); N(SiMe3)2 (3)) and [Mg{η5-C5H3-1,3-(CH2CH2NiPr2)(SiMe2NPh2)}][BPh4] (4) have been prepared and characterized. The 1H-13C HMBC and HSQC spectra of compounds 2 and 3 demonstrate the formation of 1,3-substituted cyclopentadienyl compounds and DOSY and 1H-NMR experiments support the coordination–decoordination in solution of a THF molecule in complex 2. No Schlenk-type ligand redistributions were observed in the chemical behaviour of these magnesium complexes. Compounds 2–4 are active catalysts in the hydroamination of aminoalkenes; stoichiometric reactions and kinetic measurements have been performed to gain an insight into their reaction mechanisms. A key contribution here is the catalytic reactivity of a cationic magnesium compound.

 Please wait while we load your content...
Please wait while we load your content...