Hydrogen-bond promoted nucleophilic fluorination: concept, mechanism and applications in positron emission tomography
Abstract
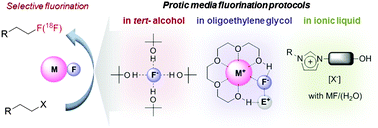
Due to the tremendous interest in carbon–fluorine bond-forming reactions, research efforts in this area have been dedicated to the development of facile processes to synthesize small fluorine-containing organic molecules. Among others, PET (Positron Emission Tomography) is one of the most important applications of fluorine chemistry. Recognizing the specific requirements of PET processes, some groups have focused on fluorination reactions using alkali metal fluorides, particularly through SN2-type reactions. However, a common “misconception” about the role of protic solvents and hydrogen bonding interactions in this class of reactions has hampered the employment of these excellent promoters. Herein, we would like to review recent discoveries in this context, showing straightforward nucleophilic fluorination reactions using alkali metal fluorides promoted by protic solvents. Simultaneous dual activation of reacting partners by intermolecular hydrogen bonding and the enhancement of the “effective fluoride nucleophilicity”, which is Nature's biocatalytic approach with the fluorinase enzyme, are the key to this unprecedentedly successful nucleophilic fluorination.
- This article is part of the themed collection: Celebrating the 75th Anniversary of the Korean Chemical Society (KCS)

 Please wait while we load your content...
Please wait while we load your content...