Peptide self-assembly: thermodynamics and kinetics
Abstract
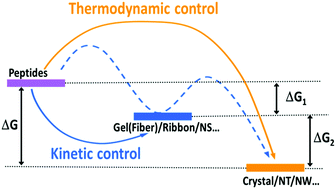
Self-assembling systems play a significant role in physiological functions and have therefore attracted tremendous attention due to their great potential for applications in energy, biomedicine and nanotechnology. Peptides, consisting of amino acids, are among the most popular building blocks and programmable molecular motifs. Nanostructures and materials assembled using peptides exhibit important potential for green-life new technology and biomedical applications mostly because of their bio-friendliness and reversibility. The formation of these ordered nanostructures pertains to the synergistic effect of various intermolecular non-covalent interactions, including hydrogen-bonding, π–π stacking, electrostatic, hydrophobic, and van der Waals interactions. Therefore, the self-assembly process is mainly driven by thermodynamics; however, kinetics is also a critical factor in structural modulation and function integration. In this review, we focus on the influence of thermodynamic and kinetic factors on structural assembly and regulation based on different types of peptide building blocks, including aromatic dipeptides, amphiphilic peptides, polypeptides, and amyloid-relevant peptides.

 Please wait while we load your content...
Please wait while we load your content...