Natural product modulators of transient receptor potential (TRP) channels as potential anti-cancer agents
Abstract
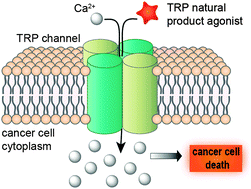
Treatment of cancer is a significant challenge in clinical medicine, and its research is a top priority in chemical biology and drug discovery. Consequently, there is an urgent need for identifying innovative chemotypes capable of modulating unexploited drug targets. The transient receptor potential (TRPs) channels persist scarcely explored as targets, despite intervening in a plethora of pathophysiological events in numerous diseases, including cancer. Both agonists and antagonists have proven capable of evoking phenotype changes leading to either cell death or reduced cell migration. Among these, natural products entail biologically pre-validated and privileged architectures for TRP recognition. Furthermore, several natural products have significantly contributed to our current knowledge on TRP biology. In this Tutorial Review we focus on selected natural products, e.g. capsaicinoids, cannabinoids and terpenes, by highlighting challenges and opportunities in their use as starting points for designing natural product-inspired TRP channel modulators. Importantly, the de-orphanization of natural products as TRP channel ligands may leverage their exploration as viable strategy for developing anticancer therapies. Finally, we foresee that TRP channels may be explored for the selective pharmacodelivery of cytotoxic payloads to diseased tissues, providing an innovative platform in chemical biology and molecular medicine.
- This article is part of the themed collections: Celebrating the ChemSocRev Lectureship winners, 2016 Emerging Investigators and Celebrating the 2016 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...