An absorption mechanism and polarity-induced viscosity model for CO2 capture using hydroxypyridine-based ionic liquids†
Abstract
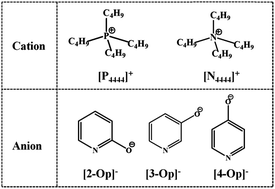
A series of new hydroxypyridine-based ionic liquids (ILs) are synthesized and applied in CO2 capture through chemical absorption, in which one IL, i.e., tetrabutylphosphonium 2-hydroxypyridine ([P4444][2-Op]), shows a viscosity as low as 193 cP with an absorption capacity as high as 1.20 mol CO2 per mol IL. Because the traditional anion–CO2 absorption mechanism cannot provide an explanation for the influences of cations and temperature on CO2 absorption capacity, herein, a novel cation-participating absorption mechanism based on the proton transfer is proposed to explain the high absorption capacity and the existence of a turning point of absorption capacity with the increase of temperature for the capture of CO2 using [P4444][n-Op] (n = 2, 3, 4) ILs. Also, the relationship between the viscosity of ILs and the linear interaction energy is proposed for the first time, which can guide how to design and synthesize ILs with low viscosity. Quantum chemistry calculations, which are based on the comprehensive analysis of dipole moment, cation–anion interaction energy and surface electrostatic potential, indicate that the different viscosities of hydroxypyridine-based ILs and the changes after CO2 absorption mainly resulted from the different distribution of negative charges in the anion.

 Please wait while we load your content...
Please wait while we load your content...