Experimental and theoretical identification of the Fe(vii) oxidation state in FeO4−†
Abstract
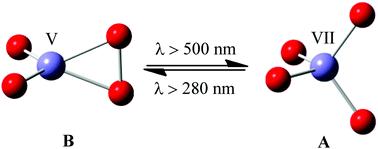
The experimentally known highest oxidation state of iron has been determined to be Fe(VI) so far. Here we report a combined matrix-isolation infrared spectroscopic and theoretical study of two interconvertible iron oxide anions: a dioxoiron peroxide complex [(η2-O2)FeO2]− with a C2v-structure and a tetroxide FeO4− with a D2d tetrahedral structure, which are formed by co-condensation of laser-ablated iron atoms and electrons with O2/Ar mixtures at 4 K. Quantum chemistry theoretical studies indicate that the Jahn–Teller distorted tetroxide FeO4− anion is a d1 species with hereto the highest iron formal oxidation state Fe(VII).


 Please wait while we load your content...
Please wait while we load your content...