Time-resolved photoelectron imaging of iodide–nitromethane (I−·CH3NO2) photodissociation dynamics†
Abstract
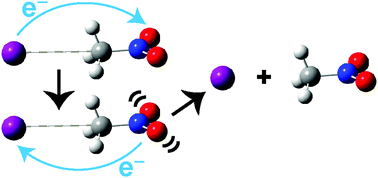
Femtosecond time-resolved photoelectron spectroscopy is used to probe the decay channels of iodide–nitromethane (I−·CH3NO2) binary clusters photoexcited at 3.56 eV, near the vertical detachment energy (VDE) of the cluster. The production of I− is observed, and its photoelectron signal exhibits a mono-exponential rise time of 21 ± 1 ps. Previous work has shown that excitation near the VDE of the I−·CH3NO2 complex transfers an electron from iodide to form a dipole-bound state of CH3NO2− that rapidly converts to a valence bound (VB) anion. The long appearance time for the I− fragment suggests that the VB anion decays by back transfer of the excess electron to iodide, reforming the I−·CH3NO2 anion and resulting in evaporation of iodide. Comparison of the measured lifetime to that predicted by RRKM theory suggests that the dissociation rate is limited by intramolecular vibrational energy redistribution in the re-formed anion between the high frequency CH3NO2 vibrational modes and the much lower frequency intermolecular I−·CH3NO2 stretch and bends, the predominant modes involved in cluster dissociation to form I−. Evidence for a weak channel identified as HI + CH2NO2− is also observed.

 Please wait while we load your content...
Please wait while we load your content...