First principles computational study on the adsorption mechanism of organic methyl iodide gas on triethylenediamine impregnated activated carbon
Abstract
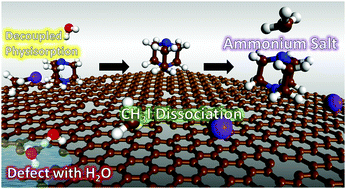
We study removal of gas-phase organic methyl iodide (CH3I) from an ambient environment via adsorption onto triethylenediamine (TEDA) impregnated activated carbon (AC). First principles density functional theory (DFT) calculations and ab-initio molecular dynamics (AIMD) simulations were extensively utilized to understand the underlying mechanism for the chemical reaction of CH3I on the surface. Our results suggest that the adsorption energy of CH3I shows substantial heterogeneity depending on the adsorption site, porosity of the AC, and humidity. It is observed that the CH3I dissociative chemisorption is largely influenced by the adsorption site and porosity. Most importantly, it is clearly shown through free energy diagrams that the impregnated TEDA not only reduces the dissociation activation barrier of CH3I but also attracts H2O molecules relieving the AC surface from poisoning by humidity, and also enhances the removal efficiency of CH3I through the chemical dissociation reaction. Our computational study can help to open new routes to design highly efficient materials for removing environmentally and biologically hazardous materials, for example radioactive iodine gas emitted following accidents at a nuclear power plant.

 Please wait while we load your content...
Please wait while we load your content...