Theoretical study on σ- and π-hole carbon⋯carbon bonding interactions: implications in CFC chemistry†
Abstract
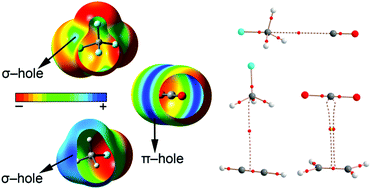
In this manuscript the ability of CO2 and several CFCs to establish noncovalent carbon⋯carbon interactions (termed as noncovalent carbon⋯carbon bonding) with atmospheric gases CO, ethene and ethyne has been studied at the RI-MP2/def2-TZVPD level of theory. We have used several CFCs (CFCl3, CF3Cl, CF2Cl2 and CH3F) and the CO2 molecule as σ- and π-hole carbon bond donors (electron poor carbon atoms). As electron rich moieties we have used CO, ethene and ethyne (electron rich carbon atom bearing molecules). We have also used Bader's theory of “atoms in molecules” to further analyse and characterize the noncovalent interactions described herein. Finally, we have analyzed possible cooperativity effects between the noncovalent carbon⋯carbon bonding and hydrogen bonding interactions in the case of ethyne.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...