A new, double-inversion mechanism of the F− + CH3Cl SN2 reaction in aqueous solution†
Abstract
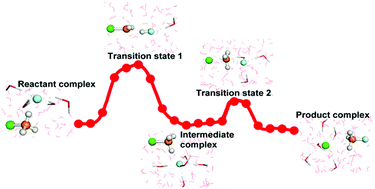
Atomic-level, bimolecular nucleophilic substitution reaction mechanisms have been studied mostly in the gas phase, but the gas-phase results cannot be expected to reliably describe condensed-phase chemistry. As a novel, double-inversion mechanism has just been found for the F− + CH3Cl SN2 reaction in the gas phase [Nat. Commun., 2015, 6, 5972], here, using multi-level quantum mechanics methods combined with the molecular mechanics method, we discovered a new, double-inversion mechanism for this reaction in aqueous solution. However, the structures of the stationary points along the reaction path show significant differences from those in the gas phase due to the strong influence of solvent and solute interactions, especially due to the hydrogen bonds formed between the solute and the solvent. More importantly, the relationship between the two double-inversion transition states is not clear in the gas phase, but, here we revealed a novel intermediate complex serving as a “connecting link” between the two transition states of the abstraction-induced inversion and the Walden-inversion mechanisms. A detailed reaction path was constructed to show the atomic-level evolution of this novel double reaction mechanism in aqueous solution. The potentials of mean force were calculated and the obtained Walden-inversion barrier height agrees well with the available experimental value.

 Please wait while we load your content...
Please wait while we load your content...