Halogen transfer through halogen bonds in halogen-bound ammonia homodimers†
Abstract
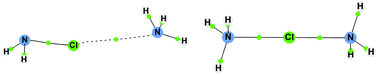
Ab initio MP2(full)/aug-cc-pVTZ calculations have been carried out to investigate the halogen transfer between haloamines and ammonia. The results show that the formation of a halogen bond complex between ammonia and the protonated N-haloamine is a preliminary step in the halogen transfer process. The complexation energies, optimized geometries, topology of electron density and potential energy surfaces for halogen transfer in these complexes have been analysed. It has been found that halogen-bound ammonia homodimers ([H3NXNH3]+, with X = Cl, Br, I) formed by interaction between NH3 and H3NX+ are symmetric, and energetically more stable than the corresponding complexes formed between NH3 and H2NX. Calculated potential energy surfaces for the transfer of the central halogen atom between the two NH3 units in [H3NXNH3]+ show single well and double well potentials for short and large N–N distances, respectively. The particular case of fluorine complexes has also been analysed. The results provide an explanation for some of the experimental facts observed for halogen transfer reactions between amines in aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...