A new understanding of the photocatalytic mechanism of the direct Z-scheme g-C3N4/TiO2 heterostructure
Abstract
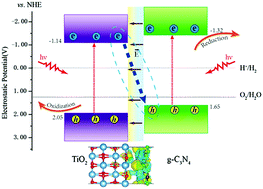
Constructing a TiO2 based heterostructure is a very effective strategy for enhancing photocatalytic performance. The details of the electronic structure, interfacial interaction, and photogenerated carrier separation are important for explaining the photocatalytic properties of a heterostructure. Herein, the density of states, charge distribution, and the band offset of the monolayer g-C3N4/TiO2 heterojunction are systematically investigated through the hybrid DFT method. Results indicated that the valence band offset and the conduction band offset of the g-C3N4/TiO2 heterostructure were 0.40 and 0.18 eV, respectively. Interfacial interaction made the TiO2 surface with negative charge, whereas the g-C3N4 surface with positive charge, which led to the formation of a built-in electric field at the interface. Under illumination, the built-in electric field accelerates the transfer of photoexcited electrons in the CB of TiO2 into the VB of g-C3N4, thus resulting in the photoexcited electrons and holes naturally accumulating in the CB of g-C3N4 and the VB of TiO2, respectively. The photoexcited electrons and holes gathering in different surface regions efficiently prolonged the lifetime of photogenerated carriers. Meanwhile, electrons in the CB of g-C3N4 and holes in the VB of TiO2 had a stronger redox ability. Therefore, g-C3N4/TiO2 is a direct Z-scheme photocatalyst, and the Z-scheme heterostructure mechanism can well explain the improved photocatalytic activity of the g-C3N4/TiO2 heterostructure.

 Please wait while we load your content...
Please wait while we load your content...