The effect of C–OH functionality on the surface chemistry of biomass-derived molecules: ethanol chemistry on Rh(100)†
Abstract
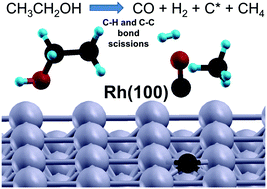
The adsorption and decomposition of ethanol on Rh(100) was studied as a model reaction to understand the role of C–OH functionalities in the surface chemistry of biomass-derived molecules. A combination of experimental surface science and computational techniques was used: (i) temperature programmed reaction spectroscopy (TPRS), reflection absorption infrared spectroscopy (RAIRS), work function measurements (Kelvin Probe – KP), and density functional theory (DFT). Ethanol produces ethoxy (CH3CH2O) species via O–H bond breaking upon adsorption at 100 K. Ethoxy decomposition proceeds differently depending on the surface coverage. At low coverage, the decomposition of ethoxy species occurs via β-C–H cleavage, which leads to an oxometallacycle (OMC) intermediate. Decomposition of the OMC scissions (at 180–320 K) ultimately produces CO, H2 and surface carbon. At high coverage, along with the pathway observed in the low coverage case, a second pathway occurs around 140–200 K, which produces an acetaldehyde intermediate via α-C–H cleavage. Further decomposition of acetaldehyde produces CH4, CO, H2 and surface carbon. However, even at high coverage this is a minor pathway, and methane selectivity is 10% at saturation coverage. The results suggests that biomass-derived oxygenates, which contain an alkyl group, react on the Rh(100) surface to produce synthesis gas (CO and H2), surface carbon and small hydrocarbons due to the high dehydrogenation and C–C bond scission activity of Rh(100).


 Please wait while we load your content...
Please wait while we load your content...