Experimental, theoretical and computational investigation of the inelastic neutron scattering spectrum of a homonuclear diatomic molecule in a nearly spherical trap: H2@C60
Abstract
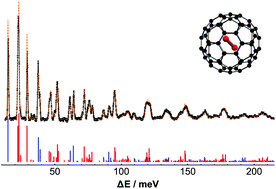
In this paper we report a methodology for calculating the inelastic neutron scattering spectrum of homonuclear diatomic molecules confined within nano-cavities of spherical symmetry. The method is based on the expansion of the confining potential into multipoles of the coupled rotational and translational angular variables. The Hamiltonian and the INS transition probabilities are evaluated analytically. The method affords a fast and computationally inexpensive way to simulate the inelastic neutron scattering spectrum of molecular hydrogen confined in fullerene cages. The potential energy surface is effectively parametrized in terms of few physical parameters comprising an harmonic term, anharmonic corrections and translation–rotation couplings. The parameters are refined by matching the simulations against the experiments and the excitation modes are identified for transfer energies up to 215 meV.

 Please wait while we load your content...
Please wait while we load your content...