Extracting nano-gold from HAuCl4 solution manipulated with electrons†
Abstract
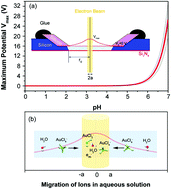
It has been fundamentally important and technologically challenging to elucidate the migration behavior of solute atoms in solvents, which can help to understand the growth of nanoparticles. Recently, ascribed to the booming development of start-of-the-art liquid environmental transmission electron microscopes (LETEMs), it has become possible to disclose, in situ, the phase segregation mechanism of elementary units in a solvent at the nanoscale. In addition, bombardment with an electron beam can induce a locally positive potential, with the application of low-conductive Si3N4 and water in LETEMs. Such merits can enable modification of the dynamic distribution and reductive behavior of the solute ions in water solutions. Herein we report the migration and segregation behaviors of Au atoms in a solvent during real time, by exploiting a charging effect in a dilute HAuCl4 water solution under electron irradiation. As a consequence, the growth kinetics of Au nanoparticles can be successfully controlled with an accelerated kinetics model. Through dynamically capturing the segregation behavior of the hydrated atoms, a resultant size-controlling mechanism is clarified with three cycles of nanoparticle growth behavior. A new insight is consequently gained into microscopically manipulating the hydrothermal synthesis of nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...