Dynamics of the conformational transitions during the dimerization of an intrinsically disordered peptide: a case study on the human islet amyloid polypeptide fragment†
Abstract
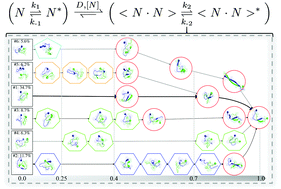
Amyloid deposits of human islet amyloid polypeptide (hIAPP) are identified in 95% of type II diabetes patients. The oligomers during the early stage of hIAPP aggregation are believed to be more cytotoxic than the mature fibrils. However, the structural details during the initial stage of hIAPP aggregation are still under debate experimentally. To understand its initial nucleation mechanism, we investigate the thermodynamics and kinetics of hIAPP(11–25) dimerization, which is the first manifestation of the interplay between intra- and inter-molecular interactions, via the construction of Markov state models from extensive molecular dynamics simulations. We identified a largely populated metastable dimer state with the antiparallel cross-β structure, although tangled coil states are also observed. The dimerization process consists of two stages kinetically: the initial collision of separate monomers followed by structural rearrangements. During the collapsing stage, hydrophobic interactions are the main driving force, although electrostatic interactions also play a role. In the subsequent structural rearrangement step, there exist heterogeneous pathways from the initial collapsed complexes to the antiparallel cross-β structure, with the transition time-scales around hundreds of microseconds. Our replica-exchange molecular dynamics simulations demonstrate that this antiparallel cross-β state is negligible in the dimer ensemble of the fibril-free S20P mutant, indicating that it is an on-pathway intermediate for hIAPP(11–25) fibrillation. These results, together with those from our previous study of the monomer, prompt us to propose a generalized model with the combination of the induced-fit and conformational-selection mechanisms for this dimerization process. These findings shed light on the understanding of hIAPP(11–25) aggregation mechanisms.

 Please wait while we load your content...
Please wait while we load your content...