Fine regulation of cellulose dissolution and regeneration by low pressure CO2 in DMSO/organic base: dissolution behavior and mechanism†
Abstract
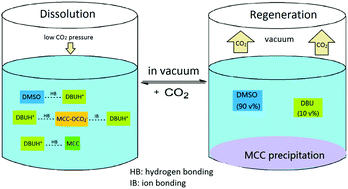
In this study, the fine regulation of the dissolution and regeneration of microcrystalline cellulose (MCC) using very low pressure (0–0.2 MPa) CO2 in a mixed solvent of dimethyl sulfoxide (DMSO) and 1,8-diazabicyclo-[5.4.0]-undec-7-ene (DBU) at a very low temperature (30 °C) was achieved. The solubility of MCC in DMSO/DBU (weight ratio of DMSO WDMSO = 0.90) could reach 9.0% at 30 °C and under CO2 pressure of 0.2 MPa. A similar phenomenon was observed in the mixed solvent DMSO/1,1,3,3-tetramethylguanidine (TMG). Moreover, ATR-FTIR, NMR, UV-Vis, TGA, XRD and DFT computational analyses were used to investigate the dissolution mechanism. It was concluded that in the mixed solvent (DMSO and organic base), DMSO helped to dissociate ion-pairs into free ions by balancing the concentration of free ions and the number of hydrogen bonds at WDMSO = 0.90. Interactions between CO2 and the solvent mixture were explored, and the results indicate that the optimum CO2 pressure not only promotes the formation of ionic bonds but also accelerates the formation of covalent bonds. In this way, these interactions prevent the MCC molecules from aggregating and facilitate the dissolving of MCC. This study gives a thorough insight into the dissolution mechanism and specificity of MCC in the CO2–DMSO/organic base solvent system, which could be helpful for the utilization and transformation of cellulose.

 Please wait while we load your content...
Please wait while we load your content...