Water bridges anchored by a C–H⋯O hydrogen bond: the role of weak interactions in molecular solvation†
Abstract
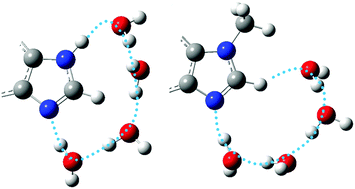
The imidazole group, characterized by an activated C(2)–H bond sandwiched between two N atoms, occurs in several biomolecules including alkaloids, amino acids, and nucleobases. The speculated role of this potential hydrogen bond donor in shaping the solvation shell around the neutral imidazole moiety, however, remains unidentified. In contrast, hydrogen bonding and electrostatic interactions are commonly observed in the imidazolium cation where the acidity of the C(2)–H bond is markedly enhanced. Here, we show direct evidence of the weakly activated C(2)–H bond in shaping the solvation shell of neutral imidazole, via spectroscopic characterization of the water clusters (Wn, n = 2–4) of two model compounds, benzimidazole (BIM) and N-methylbenzimidazole (MBIM). Infrared spectra in the OH, NH, and CH stretching regions allow unambiguous detection of a N–WW–C(2)H binding motif in the doubly hydrated cluster of both molecules. Remarkably, H-bonded water bridges between the N atom and N–H bond in BIM–W3,4 clusters are switched to the C(2)–H bond in MBIM–W3,4 with comparable binding strength, indicating that the weakly activated C(2)–H bond in the neutral imidazole moiety can serve as a potent H-bond donor.

 Please wait while we load your content...
Please wait while we load your content...