Observation of isolated ionic liquid cations and water molecules in an inert solvent
Abstract
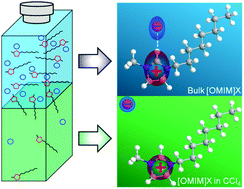
1-Octyl-3-methyl imidazolium halides ([OMIM]I and [OMIM]Cl) were loaded on top of CCl4, and an in situ inclusion process was monitored from the CCl4 phase as time elapses by infrared absorption spectroscopy. Absorption from the bands corresponding to the C(2)–H and C(4,5)–H stretch modes in the imidazolium cation was reduced significantly compared to the bulk IL spectra. This indicates that (1) the [OMIM] cation exists in CCl4 as a monomer, dissociated from the anion and other cations, and (2) hydrogen bonding between the anion and the cation increases the dipole strength of the CH moieties in the imidazolium ring. [OMIM]I was found to transfer into the CCl4 phase much faster than [OMIM]Cl, and this instigated us to compare the transfer of aqueous solutions of ionic liquids, 1-butyl-3-methyl imidazolium halides ([BMIM]I and [BMIM]Cl) into the CCl4 matrix. Not only [BMIM]I but also water molecules transferred faster compared to those in the [BMIM]Cl aqueous solution. Water molecules in CCl4 were shown to form clusters in [BMIM]I; presumably, I− anions work as nucleation centers of water clusters.

 Please wait while we load your content...
Please wait while we load your content...