Reaction mechanisms of carbon dioxide, ethylene oxide and amines catalyzed by ionic liquids BmimBr and BmimOAc: a DFT study†
Abstract
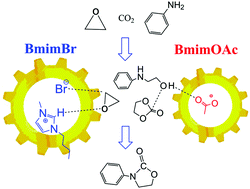
The mechanisms of the one-pot conversion of carbon dioxide, ethylene oxide, and aniline to 3-phenyl-2-oxazolidionone catalyzed by the binary ionic liquids of BmimBr and BmimOAc were explored using the DFT methods. The complex reaction above comprises of two parallel reactions and a subsequent cascade reaction. DFT calculations on reaction pathways and energy profiles reveal that the electrostatic and hydrogen-bond effects of BmimBr play a crucial role in the parallel reactions for the generation of ethylene carbonate and 2-phenylamino-ethanol. Further, the subsequent cascade reaction to generate 3-phenyl-2-oxazolidinone catalyzed by BmimOAc follows a stepwise mechanism, which is more favorable than the concerted mechanism governed by BmimBr. In addition, BmimBr can accelerate the side reaction of aniline and ethylene oxide to yield a mixture of oligomers, which accords with the experimental observation. This theoretical work provides a deep insight into the catalytic roles of binary ionic liquids and also inspires us to design high efficient catalysts for the conversion of carbon dioxide further.

 Please wait while we load your content...
Please wait while we load your content...