Spectroscopic investigations of high-energy-density plasma transformations in a simulated early reducing atmosphere containing methane, nitrogen and water
Abstract
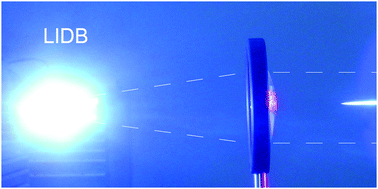
Large-scale plasma was created in gas mixtures containing methane using high-power laser-induced dielectric breakdown (LIDB). The composition of the mixtures corresponded to a cometary and/or meteoritic impact into the early atmosphere of either Titan or Earth. A multiple-centimeter-sized fireball was created by focusing a single 100 J, 450 ps near-infrared laser pulse into the center of a 15 L gas cell. The excited reaction intermediates formed during the various stages of the LIDB plasma chemical evolution were investigated using optical emission spectroscopy (OES) with temporal resolution. The chemical consequences of laser-produced plasma generation in a CH4–N2–H2O mixture were investigated using high resolution Fourier-transform infrared absorption spectroscopy (FTIR) and gas selected ion flow tube spectrometry (SIFT). Several simple inorganic and organic compounds were identified in the reaction mixture exposed to ten laser sparks. Deuterated water (D2O) in a gas mixture was used to separate several of the produced isotopomers of acetylene, which were then quantified using the FTIR technique.


 Please wait while we load your content...
Please wait while we load your content...