Contrasting reactions of hydrated electron and formate radical with 2-thio analogues of cytosine and uracil†
Abstract
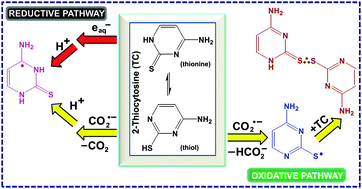
2-Thiocytosine (TC) and 2-thiouracil (TU) were subjected to hydrated electron (eaq−), formate radical (CO2˙−) and 2-hydroxypropan-2-yl radical ((CH3)2˙COH) reactions in aqueous medium. Transients were characterized by absorption spectroscopy and the experimental findings were rationalized by DFT calculations at LC-ωPBE and M06-2X levels using a 6-311+G(d,p) basis set and SMD solvation. In eaq− reactions, a ring N-atom protonated radical of TC and an exocyclic O-atom protonated radical of TU were observed via addition of eaq− and subsequent protonation by solvent molecules. However, two competing but simultaneous mechanisms are operative in CO2˙− reactions with TC and TU. The first one corresponds to formations of N(O)-atom protonated radicals (similar to eaq− reactions); the second mechanism led to 2 center–3 electron, sulfur–sulfur bonded neutral dimer radicals, TCdim˙ and TUdim˙. DFT calculations demonstrated that H-abstraction by CO2˙− from TC(TU) results in S-centered radical which upon combination with TC(TU) provide the dimer radical. In some cases, DFT energy profiles were further validated by CBS-QB3//M06-2X calculations. This is the first time report for a contradictory behavior in the mechanisms of eaq− and CO2˙− reactions with any pyrimidines or their thio analogues.


 Please wait while we load your content...
Please wait while we load your content...