Understanding positive and negative deviations in polarity of ionic liquid mixtures by pseudo-solvent approach†
Abstract
Physico-chemical properties of liquid mixtures in general display large deviations from linear behaviour, arising out of complex specific and non-specific intermolecular interactions. The polarity of liquid mixtures displaying large positive and negative deviations can be minimized and linear mixing can be achieved in liquids using a pseudo-solvent methodology. The work described herein is designed to investigate the influence of different physical parameters on the linear pseudo-solvent composition in ionic liquid mixtures. For this purpose, we have determined the deviations from linearity, ΔENT values (defined as 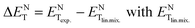 given by
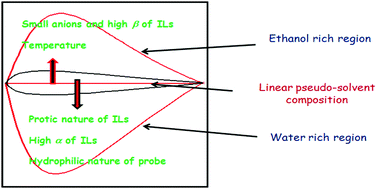
given by 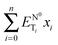 ) for binary mixtures of a variety of ionic liquids, including two molecular solvents, DMSO and formamide. Firstly, the investigations were carried out in three 1-butyl-3-methylimidazolium cation based aprotic ionic liquids and the roles of anionic structure and hydrogen bond acceptor basicities (β values) of the ionic liquids were determined. The influence of the cationic structure, i.e., the hydrogen bond donor acidity (α values) and non-associative nature of the ionic liquids, was determined using C2-methylated analogs, 1-butyl-2,3-dimethylimidazolium cation based ionic liquids. The role of the protic nature of ionic liquids was studied in two protic ionic liquids, viz., 1-methylimidazolium formate and 1-methylimidazolium acetate. The effects of the temperature, pseudo-solvent structure and solvatochromic probe structure on the ΔENT values were also explored.
) for binary mixtures of a variety of ionic liquids, including two molecular solvents, DMSO and formamide. Firstly, the investigations were carried out in three 1-butyl-3-methylimidazolium cation based aprotic ionic liquids and the roles of anionic structure and hydrogen bond acceptor basicities (β values) of the ionic liquids were determined. The influence of the cationic structure, i.e., the hydrogen bond donor acidity (α values) and non-associative nature of the ionic liquids, was determined using C2-methylated analogs, 1-butyl-2,3-dimethylimidazolium cation based ionic liquids. The role of the protic nature of ionic liquids was studied in two protic ionic liquids, viz., 1-methylimidazolium formate and 1-methylimidazolium acetate. The effects of the temperature, pseudo-solvent structure and solvatochromic probe structure on the ΔENT values were also explored.

 Please wait while we load your content...
Please wait while we load your content...