Methanol dimer formation drastically enhances hydrogen abstraction from methanol by OH at low temperature†
Abstract
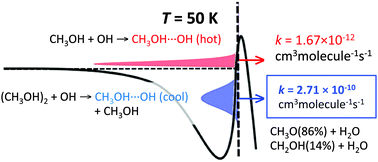
The kinetics of the reaction of methanol with hydroxyl radicals is revisited in light of the reported new kinetic data, measured in cold expansion beams. The rate constants exhibit an approximately 102-fold increase when the temperature decreases from 200 to 50 K, a result that cannot be fully explained by tunneling, as we confirm by new calculations. These calculations also show that methanol dimers are much more reactive to hydroxyl than monomers and imply that a dimer concentration of about 30% of the equilibrium concentration can account quantitatively for the observed rates. The assumed presence of dimers is supported by the observation of cluster formation in these and other cold beams of molecules subject to hydrogen bonding. The calculations imply an important caveat with respect to the use of cold expansion beams for the study of interstellar chemistry.

 Please wait while we load your content...
Please wait while we load your content...