Polarization-driven catalysis via ferroelectric oxide surfaces†
Abstract
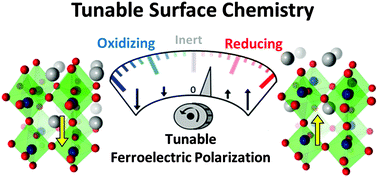
The surface chemistry and physics of oxide ferroelectric surfaces with a fixed polarization state have been studied experimentally for some time. Here, we discuss the possibility of using these materials in a different mode, namely under cyclically changing polarization conditions achievable via periodic perturbations by external fields (e.g., temperature, strain or electric field). We use Density Functional Theory (DFT) and electronic structure analysis to understand the polarization-dependent surface physics and chemistry of ferroelectric oxide PbTiO3 as an example of this class of materials. This knowledge is then applied to design catalytic cycles for industrially important reactions including NOx direct decomposition and SO2 oxidation into SO3. The possibility of catalyzing direct partial oxidation of methane to methanol is also investigated. More generally, we discuss how using ferroelectrics under cyclically changing polarization conditions can help overcome some of the fundamental challenges facing the catalysis community such as the limitations imposed by the Sabatier principle and scaling relations.

 Please wait while we load your content...
Please wait while we load your content...