A salt bridge turns off the foot-pocket in class-II HDACs†
Abstract
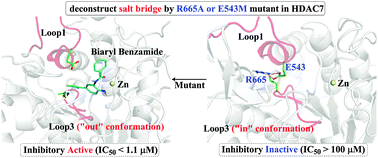
Histone Deacetylases (HDACs) are promising anticancer targets and several selective inhibitors have been created based on the architectural differences of foot-pockets among HDACs. However, the “gate-keeper” of foot-pockets is still controversial. Herein, it is for the first time revealed that a conserved R–E salt bridge plays a critical role in keeping foot-pockets closed in class-II HDACs by computational simulations. This finding is further substantiated by our mutagenesis experiments.

 Please wait while we load your content...
Please wait while we load your content...