Mechanistic investigations of CO-photoextrusion and oxidative addition reactions of early transition-metal carbonyls: (η5-C5H5)M(CO)4 (M = V, Nb, Ta)†
Abstract
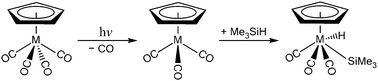
The mechanisms for the photochemical Si–H bond activation reaction are studied theoretically using a model system of the group 5 organometallic compounds, η5-CpM(CO)4 (M = V, Nb, and Ta), with the M06-2X method and the Def2-SVPD basis set. Three types of reaction pathways that lead to final insertion products are identified. The structures of the intersystem crossings, which play a central role in these photo-activation reactions, are determined. The intermediates and transitional structures in either the singlet or triplet states are also calculated to provide a mechanistic explanation of the reaction pathways. All of the potential energy surfaces for the group 5 η5-CpM(CO)4 complexes are quite similar. In particular, the theoretical evidence suggests that after irradiation using light, η5-CpM(CO)4 quickly loses one CO ligand to yield two tricarbonyls, in either the singlet or the triplet states. The triplet tricarbonyl 16-electron intermediates, ([η5-CpM(CO)3]3), play a key role in the formation of the final oxidative addition product, η5-CpM(CO)3(H)(SiMe3). However, the singlet counterparts, ([η5-CpM(CO)3]1), play no role in the formation of the final product molecule, but their singlet metal centers interact weakly with solvent molecules ((Me3)SiH) to produce alkyl-solvated organometallic complexes, which are observable experimentally. This theoretical evidence is in accordance with the available experimental observations.

 Please wait while we load your content...
Please wait while we load your content...