The reaction of CF2Cl2 with gas-phase hydrated electrons†
Abstract
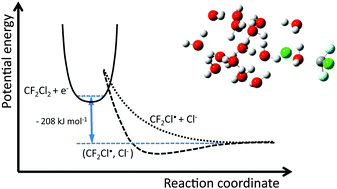
The reaction of dichlorodifluoromethane (CF2Cl2) with hydrated electrons (H2O)n− (n = 30–86) in the gas phase was studied using Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometry. The hydrated electron reacts with CF2Cl2, forming (H2O)mCl− with a rate constant of (8.6 ± 2.2) × 10−10 cm3 s−1, corresponding to an efficiency of 57 ± 15%. The reaction enthalpy was determined using nanocalorimetry, revealing a strongly exothermic reaction with ΔHr(CF2Cl2, 298 K) = −208 ± 41 kJ mol−1. The combination of the measured reaction enthalpy with thermochemical data from the condensed phase yields a C–Cl bond dissociation enthalpy (BDE) ΔHC–Cl(CF2Cl2, 298 K) = 355 ± 41 kJ mol−1 that agrees within error limits with the predicted values from quantum chemical calculations and published BDEs.


 Please wait while we load your content...
Please wait while we load your content...