Catenation of carbon in LaC2 predicted under high pressure†
Abstract
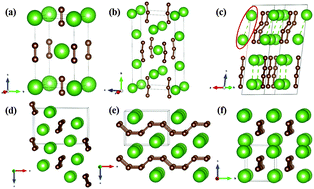
Carbon has the capability of forming various bonding states that affect the structures and properties of transition metal carbides. In this work, structural search was performed to explore the structural diversity of LaC2 at pressures of 0.0–30.0 GPa. Five stable structures of LaC2 reveal a variety of carbon structural units ranging from a dimer to bent C3, zigzag C4 and armchair polymer chains. A series of pressure-induced structural transformations are predicted, I4/mmm (i.e. experimental α phase) → C2/c → Pnma → Pmma, which involve the catenation of carbon from a dimer to zigzag C4 units and further to armchair polymer chains. The bent C3 unit appears in a novel Immm structure. This structure is the theoretical ground state of LaC2 under ambient conditions, but is kinetically inaccessible from the experimental α phase. LaC2 becomes thermodynamically metastable relative to La2C3 + diamond above 17.1 GPa, and eventually decomposes into constituent elements above 35.6 GPa. The presented results indicate that catenation of carbon can be realized even in simple inorganic compounds under nonambient conditions.


 Please wait while we load your content...
Please wait while we load your content...