A neutron scattering and modelling study of aqueous solutions of tetramethylammonium and tetrapropylammonium bromide†
Abstract
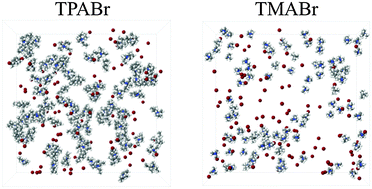
We have investigated the properties in water of two tetraalkylammonium bromides (tetramethylammonium, TMA+, and tetrapropylammonium, TPA+), at 0.4 M, using neutron scattering coupled with empirical potential structure refinement to arrive at an atomistic description. Having both a polar and an apolar moiety, it is of interest to determine the strength of each moiety as a function of the alkyl chain length. TMA+ and TPA+, having different impact as structure directors in zeolite synthesis, were chosen for this study. Water arranges tetrahedrally around TMA+ and in an almost featureless manner around TPA+. TMA+ and TPA+ show an apolar hydration with TPA+ being slightly more apolar. TPA+ has a tendency to form small clusters of 2–4 molecules and to fold into a compact configuration. Both molecules correlate similarly with the bromide ion but do not dissociate completely at this concentration.

 Please wait while we load your content...
Please wait while we load your content...