Comparing the catalytic strategy of ATP hydrolysis in biomolecular motors
Abstract
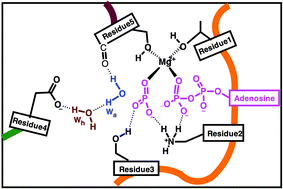
ATP-driven biomolecular motors utilize the chemical energy obtained from the ATP hydrolysis to perform vital tasks in living cells. Understanding the mechanism of enzyme-catalyzed ATP hydrolysis reaction has substantially progressed lately thanks to combined quantum/classical molecular mechanics (QM/MM) simulations. Here, we present a comparative summary of the most recent QM/MM results for myosin, kinesin and F1-ATPase motors. These completely different motors achieve the acceleration of ATP hydrolysis through a very similar catalytic mechanism. ATP hydrolysis has high activation energy because it involves the breaking of two strong bonds, namely the Pγ–Oβγ bond of ATP and the H–O bond of lytic water. The key to the four-fold decrease in the activation barrier by the three enzymes is that the breaking of the Pγ–Oβγ bond precedes the deprotonation of the lytic water molecule, generating a metaphosphate hydrate complex. The resulting singly charged trigonal planar PγO3− metaphosphate is a better electrophilic target for attack by an OaH− hydroxyl group. The formation of this OaH− is promoted by a strong polarization of the lytic water: in all three proteins, this water is forming a hydrogen-bond with a backbone carbonyl group and interacts with the carboxylate group of glutamate (either directly or via an intercalated water molecule). This favors the shedding of one proton by the attacking water. The abstracted proton is transferred to the γ-phosphate via various proton wires, resulting in a H2PγO4−/ADP3− product state. This catalytic strategy is so effective that most other nucleotide hydrolyzing enzymes adopt a similar approach, as suggested by their very similar triphosphate binding sites.
- This article is part of the themed collection: PCCP Perspectives

 Please wait while we load your content...
Please wait while we load your content...